Making the case for bar code medication preparation (BCMP) in sterile compounding by Jerry Fahrni PharmD
January 28, 2015 | In: Healthcare Barcoding Influencers
The tragic death of a hospitalized patient in Oregon [1] has once again put a spotlight on pharmacy i.v. rooms. Unfortunately this isn’t the first i.v. error to harm, or kill a patient and I’m sad to say that it probably won’t be the last. We know that IVs present higher risks than most other medications and the literature presents abundant evidence of the prevalence of pharmacy compounding errors which result in patient harm or death.2-11
According to a 1997 article by Flynn, Pearson, and Baker: A five-hospital observational study on the accuracy of preparing small and large volume injectables, chemotherapy solutions, and parenteral nutrition showed a mean error rate of 9%, meaning almost 1 in 10 products was prepared incorrectly prior to dispensing.6
The inherent problem with compounded sterile products (CSPs) is that the efficacy of IV medication administration hinges on the integrity of dose preparation and labeling in the pharmacy. If an item is compounded incorrectly in the pharmacy, no amount of verification at the bedside will alter that. Other than looking at an IV bag or syringe to ensure that no gross particulate matter is present, without chemical analysis it is impossible to verify the contents. Occasionally a color change will acknowledge the addition of the correct additive – yellow multivitamins, red doxorubicin, and so on – but even then, the correct amount (volume/dosage) cannot be verified.
The United States Pharmacopeia (USP) Chapter <797> – Pharmaceutical Compounding—Sterile Preparations states that “Compounding personnel are responsible for ensuring that CSPs are accurately identified, measured, diluted, and mixed; and are correctly purified, sterilized, packaged, sealed, labeled, stored, dispensed, and distributed. These performance responsibilities include maintaining appropriate cleanliness conditions and providing labeling and supplementary instructions for the proper clinical administration of CSPs.”12
The American Society of Health-Systems Pharmacists’ (ASHP) Guidelines on Quality Assurance for Pharmacy-Prepared Sterile Products states that “All compounding personnel, mainly pharmacists and pharmacy technicians, are responsible for compounding and dispensing sterile products of correct ingredient identity, purity, strength, and sterility and for dispensing them in appropriate containers, labeled accurately and appropriately for the end user.”13
Both USP <797> and ASHP Guidelines make it clear that the responsibility for ensuring accurate CSP preparation resides solely with the pharmacy. But how does one accomplish such a responsibility? The answer is not simple. Diligence, improved workflow, proper procedures, checklists, etc. all have a role in improving the sterile compounding process. However, all of these rely on fallible human interaction. Humans make mistakes, and there’s no way around that. Technology, when used properly can be utilized to ensure that CSPs contain both the proper ingredients and correct amounts.
Technology-assisted CSP preparation includes computerized workflow processes that require bar-code scanning of containers and ingredients, image capture, gravimetrics, optical recognition, robotics, and Telepharmacy. All these technologies have the potential to improve accuracy during CSP production, and thus improve patient safety. However, in my opinion, currently only bar-code scanning technology can positively identify an ingredienta during the compounding process. And while the use of images is a significant improvement over the syringe pull-back methodb used to “double check” amount added, only gravimetrics can be used to guarantee accurate volumes.c
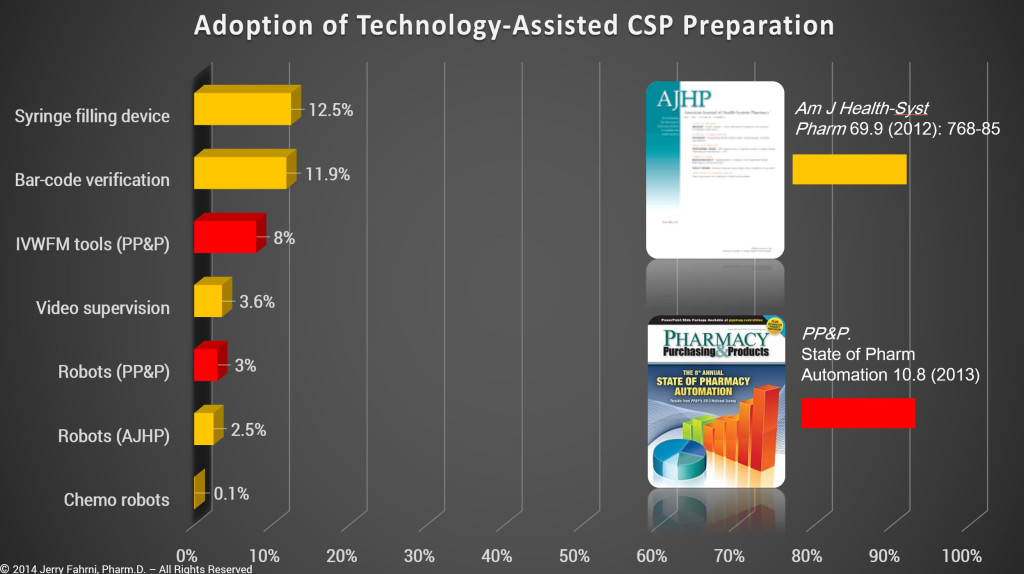
While it is true that many pharmacies apply bar-code labels to CSPs, that particular bar code is used for bar code medication administration (BCMA) at the bedside and not to verify the accuracy of the CSP. In addition, based on data taken from ASHP and PP&P surveys, we know that technology-assisted CSP preparation is not common.d
On the upside, interest in i.v. room technology has been building for the past couple of years. Pharmacy has been slow to respond to the need, but at least there appears to be progress. I was involved in several conversations around pharmacy i.v. room technology and CSP production at ASHP Midyear back in December. However, few were discussing the need for bar-code scanning during the medication preparation process. It’s one of those things that seems to get easily overlooked when discussing technology-assisted CSP preparation; perhaps because bar-code scanning remains a very manual process. Even so, I believe that the use of bar-code scanning in the i.v. room during CSP production remains one of our most valuable weapons in the fight against errors. It will be interesting to see how this technology is used over the next two or three years.
by Jerry Fahrni
…………………………………………………
Footnotes:
- potentially RFID, but existing RFID technologies require that pharmacies attach RFID tags to existing drug packages. This is often accomplished by pairing the RFID tag with the medication via the bar code, once again boiling the accuracy down to a bar code scan.
- “After injecting the medication into the container, the syringe plunger is pulled back to display the amount of medication or diluent that was added to the infusion container. This is accompanied by the actual drug or diluent container that was supposed to be added to the infusion container. In ISMP’s recently published compounding safety guidelines, this proxy method of verification of IV admixtures was strongly discouraged; this method should never be used in the preparation of chemotherapy, complex or pediatric/neonatal solutions, or compounded sterile products (CSPs) with high-alert medications.” [Hosp Pharm. Nov 2013; 48(10): 803–806.]
- I’ve had people argue that gravimetrics is not accurate when several items have similar, or the same specific gravity. This is true in theory, but not in practice. If given the same volume of two liquids with similar specific gravities and asked to identify them by weight alone, one would fail. However, it would be relatively simple to verify the volume by weight of a known drug, i.e. bar code scan the liquid for identification first, then weigh it. No system is 100% if the end user refuses to follow the proper procedure.
- Based on my personal experience, and data collected in the past 18 months, the numbers presented by ASHP and PP&P are overly optimistic. I believe that data presented in the surveys is skewed by several variables.
References:
- Wrong drug put in IV bag led to fatal Bend hospital error. 2015. Available at: http://www.ktvz.com/news/st-charles-to-issue-results-of-fatal-drugerror-probe/30115104. Accessed January 4, 2015.
- Report: Limited FDA Survey of Compounded Drug Products. 2015. Available at: http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/ucm155725.htm. Accessed January 11, 2014.
- Trissel LA. “Compounding our problems–again.” Am J Health-Syst Pharm. 1 Mar. 2003: 432.
- Selenic D, Dodson DR, Jensen B et al. “Enterobacter cloacae bloodstream infections in pediatric patients traced to a hospital pharmacy.” Am J Health-Syst Pharm. 2003; 60:1440–6.
- Niedowski E, Bor J. State to probe Hopkins death: 2-year-old cancer patient died after receiving improper IV mixture. December 20, 2003. Baltimore Sun, Baltimore, MD.
- Flynn, EA, Pearson, RE, Barker, KN. “Observational study of accuracy in compounding IV admixtures at five hospitals.” Am J Health-Syst Pharm. 1997 Apr 15; 54: 904–912
- Solomon SL, Khabbaz RF, Parker RH, et al. “An outbreak of Candida parapsilosis bloodstream infections in patients receiving parenteral nutrition.” J Infect Dis 1984; 149:98–102.
- Hughes CF, Grant AF, Leckie BD, et al. “Cardioplegia solution: A contamination crisis.” J Thorac Cardiovasc Surg 1986; 91:296–302.
- Associated Press. Pittsburgh woman loses eye to tainted drug; 12 hurt. Baltimore Sun. November 9, 1990:3A.
- Dugleaux G, Coutour XL, Hecquard C, et al. “Septicemia caused by contaminated parenteral nutrition pouches: The refrigerator as an unusual cause.” J Parenter Enteral Nutr 1991; 15:474–475.
- Perrin J. “Unsafe activities of compounding pharmacists.” Am J Health-Syst Pharm 1995;52:2827–2828.
- cn, (2014). PHARMACEUTICAL COMPOUNDING STERILE PREPARATIONS – RESPONSIBILITY OF COMPOUNDING PERSONNEL. [online] Available at: http://www.pharmacopeia.cn/v29240/usp29nf24s0_c797s3.html [Accessed 21 Feb. 2014]
- American Society of Health-System Pharmacists. ASHP Guidelines on Compounding Sterile Preparations. Am J Health-Syst Pharm. 2014; 71:145–66.
- Cohen M, Smetzer J. ISMP Medication Error Report Analysis – Leucovorin-Levoleucovorin Mix-Up; Two Error-Reduction Principles, One Change; Syringe Pull-Back Method of Verifying IV Admixtures Is Unreliable; Fleet Enema Saline Is Not Just Saline; ISMP Processes Health IT Error Reports.Hospital Pharmacy. 2013;48(10):803-806. doi:10.1310/hpj4810-803.
